Class Action Lawsuit Against AstraZeneca for Vaccine Injury Moves Forward

Pharmaceuticals giant says ‘benefits of vaccination outweigh the risks of extremely rare potential side effects.’
AstraZeneca is facing a landmark legal case in the High Court over allegations of injuries caused by its COVID-19 vaccine.
The Telegraph reported on Wednesday that lawyers could file legal cases in the High Court by the end of the year in a class action against the Anglo-Swedish pharmaceutical company AstraZeneca, representing at least 80 people.
People have died as a result of VITT (Vaccine-Induced Thrombosis and Thrombocytopenia) caused by the AstraZeneca vaccine, Vaxzevria, which was developed in collaboration with Oxford University.
Others claim to have been injured by the long-term debilitating condition.
Lawyers claim that there was no warning of the risk of VITT in the product information on the date the vaccine was supplied.
The legal documents could not be independently verified by The Epoch Times.
According to the Epoch Times, Peter Todd, a consultant solicitor with Scott-Moncrieff & Associates, initiated a claim against AstraZeneca under the Consumer Protection Act 1987, representing dozens of people who claim that their loved ones experienced severe reactions or died after receiving the vaccine.
At the time, Mr. Todd told The Epoch Times that it “remains to be seen whether AstraZeneca will be disputing that the vaccine was the cause of his death.”
“Even if they don’t contest this, I expect them to contest civil liability for his death.” “That is a legal question for the court to resolve in due course,” he added.
1.2 Per Cent
According to The Telegraph, the case also hinges on the efficacy of the COVID-19 vaccine and claims that it is “defective.”
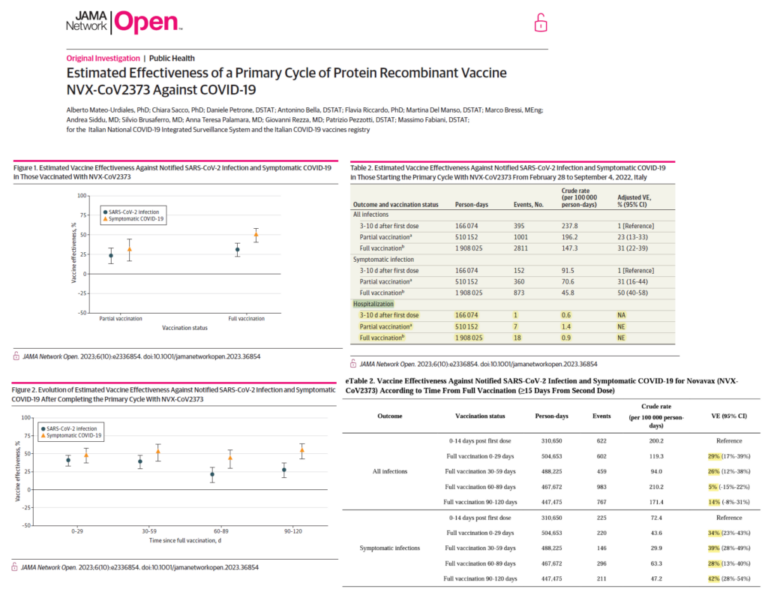
Following clinical trials, AstraZeneca issued press releases stating that the vaccine was between 62% and 90% effective at preventing symptomatic Covid-19, with an average of 70%.
However, according to the legal claim, “the absolute risk reduction for Covid-19 prevention was only 1.2 percent.”
AstraZeneca responded by telling The Epoch Times that authorities granted full marketing approval based on the “safety profile and efficacy” of the vaccine.
Claire Hibbs, a spokeswoman for Vaccine, Injured, Bereaved UK (VIB UK), has been unable to work since developing vaccine-induced immune thrombocytopenia and thrombosis (VITT) after receiving the AstraZeneca jab in 2021.
She told The Epoch Times that “we’re finally going to be listened to.”
“We can now go public about what AstraZeneca has done to us.” “Hopefully, this will get some attention because we’ve been ignored for so long,” she said, adding that they are raising funds for legal fees through crowdfunding.
Several VIB UK members are still waiting for financial assistance from England’s Vaccine Damage Payment Scheme, which is limited to a single, lump-sum payment of £120,000 ($136,000).
Some have been denied because they have been diagnosed with VITT but do not meet the strict 60 percent level of disablement benchmark.
‘Our Sympathy Goes Out’
According to an Astrazena spokesperson, “patient safety is our highest priority, and regulatory authorities have clear and stringent standards to ensure the safe use of all medicines, including vaccines.”
“Our sympathy goes out to anyone who has lost loved ones or reported health problems,” he went on to say.
“From the body of evidence in clinical trials and real-world data, Vaxzevria has continuously been shown to have an acceptable safety profile and regulators around the world consistently state that the benefits of vaccination outweigh the risks of extremely rare potential side effects,” he went on to say.
According to him, the Medicines and Healthcare products Regulatory Authority (MHRA) “has granted full marketing approval for Vaxzevria for the UK based on the safety profile and efficacy of the vaccine.”
AstraZeneca reported on Wednesday that revenue from its COVID-19 treatments had dropped to zero in the previous quarter.