Health Implications of Poor COVID-19 mRNA Testing: Miscarriage, Vision Loss, Immunotoxicity

In this series, “Promise or Peril: Alarming COVID-19 mRNA Vaccine Issues,” we look at how the introduction of mRNA technology lacked an adequate regulatory framework, which set the stage for serious adverse events and other concerns related to insufficient safety testing of lipid nanoparticles, spike protein, residual DNA and lipid-related impurities, as well as truncated/modified mRNA species.
Summary of Key Facts:
- The COVID-19 mRNA vaccines were approved for human use without extensive animal testing on the active ingredients.
- The animal testing data, which we thoroughly review, show that the mRNA and lipid nanoparticle (LNP) shell are present in tissues at high concentrations for approximately 72 hours, which is consistent with the timing of systemic reactions to vaccination occurring within the first two to three days.
- Because of their long half-life, the lipids used to create the LNP shell are likely to remain in the body for four to five months. (pdf)
- Details on animal testing reveal an increase in adverse event rates, such as joint inflammation and early pregnancy loss.
- By January 2021, regulatory authorities were aware of the adverse events discovered in animals. Nonetheless, the products were approved for use in humans, with the assumption that the benefits would outweigh the risks.
- Post-licensure research on rare adverse events has discovered evidence of post-vaccination myocarditis, neurological issues, thrombocytopenia (low platelet counts), and clotting, and a new study shows a strong, twofold increased risk of vision loss.
False Reassurances From CDC
As mentioned in Part 1, the U.S. The FDA did not require Pfizer and Moderna to conduct biodistribution testing on the active mRNA encoding spike protein used in the vaccines. Because of the FDA’s lax biodistribution and pharmacokinetic testing, a “bioengineered egg” was approved based solely on an examination of the shell; the contents were not tested.
Pfizer’s January 2021 reports to the FDA (pdf), Australian, and Japanese health authorities, as well as the European Medicines Agency (EMA) (pdf), include identical animal data demonstrating how the new lipid nanoparticle (LNP) shell travels throughout the body. The reports also demonstrate how a substitute (“fake”) mRNA (encoding for luciferase) was used to visualize mRNA movement.
Despite its sinister name, luciferase is a harmless enzyme found in fireflies and elsewhere in nature that is bioluminescent—that is, it glows in the dark. Luciferase can thus be used to visualize biological compounds in animal tissues.
According to these reports, the LNP shell has dispersed widely. Based on scant data on animals and no human data on biodistribution, the Centers for Disease Control and Prevention (CDC) provided false assurances about the duration of biological activity and distribution. People believed these statements to be true. As a result, when data contradicting these claims was available at the time, the agency issued vague and misleading assurances.
Although the CDC’s original webpage, which is now only available through web archives, stated that mRNA is broken down within days and that the spike protein does not persist in the body for more than a few weeks—we simply did not have this data, leading them to remove the highlighted duration reference.
To comprehend the health implications of the new mRNA vaccine technology, we must first investigate where luciferase, spike protein, and LNPs travel in the body and how long they remain.
What Happens to the ‘Glow-in-the-Dark’ Luciferase?
The European Medicines Agency (EMA) is the FDA of the European Union. The EMA report (pdf) provides more information about animal studies that show where the luciferase and LNP shell travel, as well as the concentrations of these compounds in tissues immediately following vaccination.
Pfizer conducted a study in which they used two methods to tag the mRNA for luciferase and the LNP shell for radioactivity.
Within six hours, the luciferase mRNA signals at the injection site reached 10,000 times that of the control animals. Lymph nodes and the liver were also found to have luciferase activity. The signal then gradually faded over the next 72 hours. (page 46) Levels continued to fall, and by day nine, the experimental animals’ luciferase levels were seven times those of the control animals. After day nine, no additional measures were taken. This increased luciferase activity in the first 72 hours correlates with potential side effects from vaccination.
The LNP shell radioactivity study discovered the highest levels in most tissues 8 to 48 hours after injection. The liver held the majority of the LNP. The report then describes how long the lipids remain in the liver. When the data is extrapolated to humans, one of the lipids (ALC-0315) may remain in the liver for four to five months. (pages 53-54)
These two animal studies show that mRNA and the LNP shell can be found at peak concentrations in tissues within 72 hours.
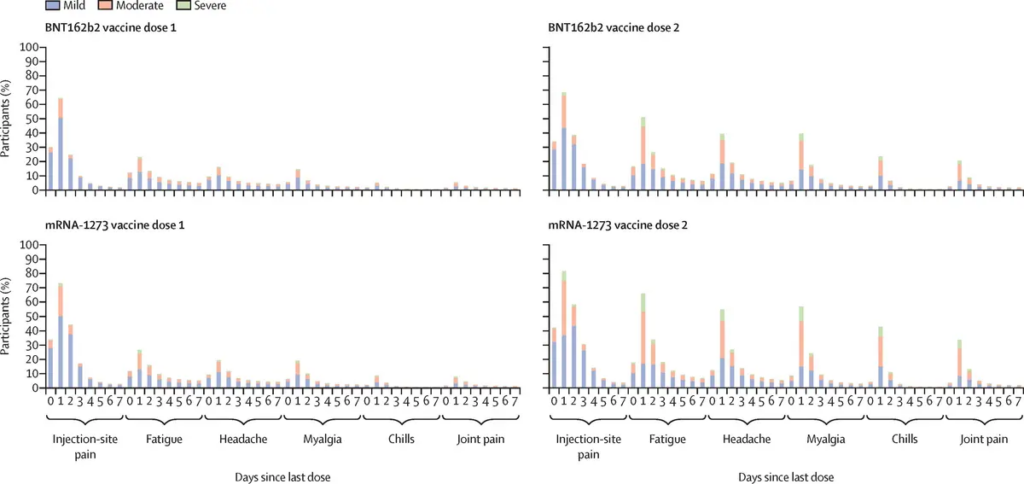
Figure 1. Vaccination Adverse Reactions by Days Since Last Dose
What Becomes of the Spike Protein?
Spike protein is produced and displayed on the surface of our cells after the vaccine LNP enters our cells and releases the mRNA. The mRNA code contains instructions for producing the full-length spike protein, which consists of two subunits (S1 and S2). When the immune system detects spike protein-positive cells, it reacts as if the virus has infected the cell.
When the immune system destroys these cells, antibodies that bind to the spike protein are produced. However, the spike protein, including its S1 subunit, can be released into the bloodstream during the immune system’s messy process of cell digestion.
Circulation of the spike protein in the blood may explain some serious adverse events, such as myocarditis and other adverse events, such as rare neurological issues caused by COVID-19 infection and vaccination with BNT162b2 and mRNA-1273.
According to one study published in Circulation, “markedly elevated levels of full-length spike protein (33.9222.4 pg/mL), unbound by antibodies, were detected in the plasma of individuals with post-vaccine myocarditis, whereas no free spike was detected in asymptomatic vaccinated control subjects (unpaired t-test; P0.0001).”
This study suggested that the difference was likely due to overactive innate immunity rather than neutralizing antibodies in the myocarditis group. In other words, having an excess of antibodies did not cause myocarditis. The authors believe that another component of the immune system, innate immunity, is at work.
Innate immunity is what helps the body fight infection, even when it encounters a new virus or bacteria for the first time. To identify foreign invaders, these innate “first responders” (inflammatory cytokines) require no training. Allergies are associated with an overactive innate response, which may also be the cause of myocarditis.
In contrast, some authors have proposed that suppressing the natural innate immune response could explain some of the adverse events associated with the COVID-19 mRNA vaccine.
There was no way to predict these issues because the active mRNA and the spike protein it encoded were never tested in animals or live tissue culture. The FDA effectively approved a product without conducting adequate preclinical toxicity testing.
How Toxic Is the Lipid Nanoparticle Shell?
The EMA emphasizes that “no traditional pharmacokinetic or biodistribution studies have been performed with the vaccine candidate BNT162b2.” (page 45) The biodistribution studies are important because “ALC-0315 and ALC-0159 are novel excipients, not previously used in an approved finished product within the EU.” They are novel compounds that differ from previous research on PEGylated compounds used in a wide range of injected medications.
According to the EMA report, the lipid ALC-0315 has a relatively long half-life. (page 46) The term half-life refers to how long it takes for half of a compound to be removed. The EMA report clarifies on page 54 that we can expect half of the ALC-0315 to be removed from the human body within 20 to 30 days, and 95 percent to be removed within four to five months.
Before making a decision on authorization, the Japanese government’s Pharmaceutical Safety and Environmental Health Bureau (February 2021) reviewed all of the data in a separate deliberation summary (pdf). The report showed effects on the liver that were “considered to be of little toxicological significance.” (page 20) However, the report also notes that “long-term repeated-dose toxicity of Comirnaty has not been evaluated” and thus the dosing regimen should be limited, and the use of lipids in the vaccine should not be considered a precedent. In other words, despite the fact that PEGylated compounds have been used in medicine for many years, there is little information on their use as part of an LNP carrying a vaccine, and thus no existing data to support repeated doses, such as boosters, beyond the initial primary series.
The EMA agreed, saying, “This [BNT162b2] vaccine contains two new components (cationic lipid ALC-0315 and PEGylated liquid ALC-0159) in the LNP, for which there is limited experience.” Given the long half-life of the ALC-0315 lipid and the toxicity of the PEG-lipid ALC-0159, the EMA acknowledged that, while there are no immediate and obvious concerns for human use, “current evidence is not definitive.”
“Regarding PEG-related toxicity which is known to depend on the dose, dose frequency, duration of treatment, and molecular weight of the PEG protein, immunogenicity is not expected to be an issue due to the low molecular weight of this PEG (<2KDa). The scientific data available at this stage do not raise noticeable concerns regarding immunogenicity or immunotoxicity of the PEG, but current evidence is not definitive.” (page 134, p. 134).
The EMA reports that serious adverse events were twice as common in the vaccine group (21 percent) as in the placebo group (13 percent), with the most frequently reported system/organ/class adverse events being “general disorders and administration site conditions” (11.9 percent versus 2.9 percent), “musculoskeletal reactions” (5.5 percent versus 2.1 percent), and “nervous system disorders” (4.2 percent versus 2.1 percent).
Because the long-term effects of the active ingredient (mRNA encoding spike protein) were not tested in these animal studies, these findings likely speak to the immunotoxicity of the LNP shell itself; however, the EMA concluded that the benefits overall were positive enough to proceed, given the protection afforded to the elderly and those with comorbidities.
Inflammation and Miscarriage Rates Noted in EMA Report
Repeat dose toxicity studies in rats given one dose per week for three weeks revealed joint and lymph node inflammation, as well as changes in bone marrow (page 49).
The report states on page 50 of the reproductive toxicity tests: “There was an increase (2x) of pre-implantation loss (9.77%, compared to control 4.09%), although this was within historical control data range (5.1%-11.5%).” In other words, while the miscarriage rate was twice as high in the vaccinated animals as in the control animals, the rate was within the expected range based on previous studies.
Interestingly, the BNT162b1 candidate—one of Pfizer’s two vaccine candidates that coded for the receptor binding domain only, rather than the full-length spike—had a much lower pre-implantation loss rate (4.8 percent) than the vaccine that was eventually brought to market, while the second model (BNT162b2) was chosen for global use due to fewer side effects.
Some Types of Vision Loss Correlate with Vaccination
A new study published in Nature confirms a “strong correlation between vaccination with an mRNA vaccine and retinal vascular occlusion.” The retina receives light and converts it into nerve signals, which are translated into vision in the brain. The retina is covered with tiny blood vessels, and when these become occluded (blocked) due to poor cardiovascular health or diabetes, vision is lost.
Given the mounting evidence that vaccination causes occlusion, the authors matched 745,041 vaccinated and 3,874,458 unvaccinated people, resulting in a final comparison group of more than 500,000 individuals in each group.
Researchers discovered 415 cases among vaccinated people aged 18 to 64 years old and 1108 cases among those aged 65 or older, representing a more than threefold increased risk of retinal occlusion following vaccination within 12 weeks and a more than twofold increased risk up to two years later compared to the unvaccinated. There was no difference in risk between mRNA vaccine brands (Pfizer versus Moderna), and the risk was elevated after both doses one and two.
The researchers hypothesize that the blockage is caused by the similarity between spike proteins and human proteins.
To summarize, these reports raise a number of troubling issues, which we will address in this series.
Is the immune response to the LNP linked to post-vaccination blindness? The LNP capsule is dispersed throughout the body, mostly to the liver. Does it fall apart or stay in a capsule?
The LNP, like the S1 subunit of the SARS-CoV-2 spike protein (in mice), can cross the blood-brain barrier (BBB). What are the implications of the LNP and S1 subunits crossing the BBB?
Does the lipid nanoparticle ALC-0315, which has a long half-life (around six weeks in the liver), clear 95 percent of the lipid from the human body within four to five months as expected?
According to the EMA (page 134), there is known immunotoxicity associated with polyethylene glycol (PEG), and this is the first time that PEG-lipid ALC-0159 has been used as a vaccine component in humans.
How long does mRNA stay in the body? Does it stay in the capsule until it is released in cells? Does it fall out of the LNP capsule? What problems could mRNA cause if it circulates in the bloodstream? (Series Part 3)
How long does the spike protein stay in the body? Where does it travel? How might the spike protein or its S1 subunit cause myocarditis or other adverse events, including neurological issues? (Series Part 4)
The EMA report also noted impurities in the vaccine doses produced using the commercial manufacturing process that were not found in the clinical trial vials, including RNA fragments that may code for unexpected proteins, potentially causing adverse reactions, including allergic reactions, which we will discuss later in this series. The latter demonstrates an uncanny predominance in females, highlighting the need for tailored risk assessment along with the vaccine.
What causes peripheral nerve effects like Bell’s Palsy? What is the most recent research describing neurologic, cardiac, autoimmune, and reproductive adverse events? Is there anything people can do to reduce their risk of a future adverse event?
The adverse events noted by the EMA in January 2021 suggest that more thorough animal testing and additional biodistribution and safety studies in humans should have been performed before licensing these products. Furthermore, the EMA report noted that differences in batches were discovered, and additional reports were requested from the manufacturer. Where is the public communication regarding updates on these issues?
The FDA-requested post-marketing surveillance does not require submission of reports on the incidence and natural course of vaccine myocarditis until September 2024, despite the fact that required studies could have been performed even within the compressed timeframe. Furthermore, given the massive earnings these products have garnered from global distribution, why are the post-marketing studies not accelerated?
In Part 3, we investigate how the design of the LNPs may predispose them to clustering or falling apart, both of which can lead to clotting.