Oncologist: C19 Vaccine-Induced “Turbo Cancers” May Be Treatable By Popular Sleep Medication
Dr. William Makis reviews eight recent peer-reviewed papers on melatonin and cancer.
Papers Examined:
Putta et al., 2023 Sep – Melatonin: Cancer Therapy Pathways and Nanotechnological Advancements
Megerian et al., 2023 Jun – Melatonin and Prostate Cancer: Anti-tumor Roles and Therapeutic Application
Davoodvandi et al., 2022 Sep – Melatonin and cancer suppression: insights into its effects on DNA methylation
2022 Aug – Florido et al – Melatonin Induces ROS Production in Cancer Cells: Understanding the Mechanism of Action
Monayo et al., 2022 May – The Prospective Use of Melatonin in the Treatment of Epigenetic Dysfunctional Diseases
Leilei Wang et al., 2022 Mar – Melatonin Use in Cancer Treatment: Where Are We?
Gonzalez et al., 2021 Jul – Melatonin as an Adjuvant to Antiangiogenic Cancer Treatments
Talib et al., 2021 Apr – Melatonin in Cancer Treatment: Current Knowledge and Future Prospects
2023 Sep – Putta et al – Melatonin: Avenues in cancer therapy and its nanotechnological advancements
“According to clinical investigations, melatonin has the potential to prevent and cure cancer”
Melatonin is essential for tumor growth. Melatonin deficiency hastens tumor development.
It can treat a variety of cancers in vivo at normal dose levels ranging from 10 to 50 mg/day (breast, lung, colon, liver, gastric, glioblastoma, ovarian, cervical, prostate, skin).
There is compelling evidence that it reduces side effects while increasing the therapeutic benefits of chemotherapy and radiation.
Melatonin has a limited ability to penetrate mucosal and dermal barriers, has a shorter half-life, and is rapidly eliminated from blood circulation due to its chemical properties.
Melatonin’s use is currently restricted due to its unfavorable pharmacokinetic properties.
Melatonin nanoparticles are being developed in order to increase its use in cancer treatment.
2023 Jun – Megerian et al – Melatonin and Prostate Cancer: Anti-tu
Exogenous factors that interfere with normal pineal secretory activity, such as aging, poor sleep, and artificial light at night, have been linked to an increased risk of prostate cancer.
Melatonin secretion becomes circadian between the ages of one and three years and declines by 10-15% per decade after that.
Diminished lifetime melatonin secretion has been proposed as a trigger for not only aging (dubbed “Age Clock”), but also age-related conditions such as cancer.
Cancer risk is increased when the circadian rhythm is disrupted.
The relationship between nighttime light exposure and the risk of prostate cancer
Several studies have linked non-standard shift work and shift work sleep disorder to poor health outcomes, most notably infertility, lower urinary tract symptoms, and prostate cancer in men.
Rotating shift workers who alternate between a day and/or afternoon shift and a night shift have a significantly increased risk of prostate cancer.
Melatonin’s influence on prostate cancer cells:
Melatonin protects against carcinogenesis by lowering genomic instability.
Melatonin inhibits cancer cell glucose metabolism.
Melatonin inhibits DNA replication and cell proliferation in cancer cells.
Melatonin inhibits cell proliferation and promotes apoptosis by downregulating NF-kB activity.
Melatonin inhibits angiogenesis.
Melatonin reduces the activity of androgen receptors.
Melatonin inhibits metastasis by inhibiting matrix metalloproteinases, which help cancer cells spread.
Melatonin inhibits cancer initiation and progression by modulating mitochondrial activity and function (melatonin improves mitochondrial function by inhibiting the Akt/mTOR signaling pathway).
Melatonin inhibits the production of inflammatory cytokines such as IL-17, which promote cancer.
Melatonin has anti-cancer properties because it resynchronizes the circadian rhythm.
Previous clinical trials that investigated the anti-tumor effects of melatonin used a maintenance dose of 20 mg per day for several weeks to years (Gonzalez et al).
“Considering that there were no major side effects related to melatonin except for minor psychological or neurocognitive problems (Foley et al), we recommend a higher dosage for a definite anti-tumor activity.”
2022 Sep – Davoodvandi et al– Melatonin and cancer suppression:
Lerner and colleagues discovered melatonin (N-acetyl-5-methoxy-tryptamine) as an endogenous hormone in bovine pineal tissue in 1958.
Melatonin biosynthesis occurs in bone marrow and lymphocytes, the gastrointestinal (GI) tract, the eyes, and possibly every cell.
Melatonin is synthesized in the mitochondria of eukaryotic cells.
There is a strong link between abnormal DNA methylation and cancer incidence.
Epigenetic changes over large chromatin regions in cancer cause epigenetic instability and subsequent gene expression changes.
Disrupted epigenetic alterations in cancer cells, such as abnormal DNA methylation, can result in tumor cell heterogeneity and a poor prognosis.
Cancer epigenetic modifications are classified into three major processes: non-coding RNAs, histone modification, and DNA methylation.
Melatonin significantly altered the status of DNA methylation, particularly in breast cancer tissue.
As a result, the changes in DNA methylation inhibited cancer cell proliferation, progression, and metastasis.
Melatonin was found to reverse chemo-resistance to current drug regimens.
2022 Aug – Florido et al – Melatonin Induces ROS Production in Cancer Cells: Understanding the Mechanism of Action
Melatonin is produced by the pineal gland and several tissues (including the skin, gastrointestinal system, bone marrow, and lymphocytes).
Melatonin is produced in greater quantities by mitochondria than by other cellular compartments.
Melatonin participates in numerous cellular processes and is a powerful free radical scavenger and broad-spectrum antioxidant.
“Melatonin is of particular importance in the development of innovative cancer treatments due to its oncostatic impact and lack of adverse effects”
Cancer-fighting properties:
Melatonin induces ROS (Reactive Oxygen Species) production in cancer cells via multiple pathways, resulting in cancer cell apoptosis.
Melatonin increases antioxidant enzyme activity by controlling gene expression.
Melatonin is a hormone that regulates mitochondrial homeostasis.
Melatonin is effective in all three stages of tumorigenesis: cancer initiation, progression, and metastasis.
Melatonin prevents cancer initiation by scavenging free radicals, inhibiting metal-induced DNA damage, stimulating antioxidant enzymes, enhancing DNA repair, and suppressing pro-oxidative enzymes.
Melatonin slows cancer progression by impairing proliferation and angiogenesis.
Melatonin inhibits hypoxia-induced cancer cell migration.
Monayo et al., 2022 May – The Prospective Use of Melatonin in the Treatment of Epigenetic Dysfunctional Diseases
Because of the broad spectrum of action of melatonin, epigenetic modification has been one of the fundamental tools used to elucidate the possible mechanisms involved by this drug in a variety of cancer types.
Melatonin inhibits tumor growth and metastasis by decreasing NF-B activation via phosphorylation.
MicroRNAs (non-coding RNA):
Melatonin raises levels of tumor-suppressing microRNAs.
Melatonin increased the levels of miR-152-3p, which reduces cell proliferation, angiogenesis, and cancer cell invasion (as seen in glioblastoma cells).
Melatonin increased miR-152-3p levels in breast cancer cells.
Melatonin also increased miR-16-5p levels, which suppresses gastric cancer as well as breast, ovarian, cervical, hepatic, prostate, bone, and lymphatic cancers.
Melatonin inhibits the oncogenic microRNA miR-483, which has been linked to thyroid cancer, breast cancer, ovarian cancer, colorectal cancer, hepatocellular cancer, and pancreatic cancer.
Melatonin inhibits the oncogenic microRNA miR-155, which inhibits the growth of glioblastoma cells.
Light produced artificially:
Modern artificial lights, particularly those emitting blue light, have been linked to a slew of health issues, including cancer.
Artificial light, for example, can cause circadian disruptions by desynchronizing the circadian pacemaker (SCN), resulting in lower levels of melatonin secretions from the pineal gland.
The effects of artificial light at night on hormonal imbalances (lower melatonin) and impaired circadian rhythms can lead to cancer.
Melatonin reduces the risk of breast, prostate, liver, and skin cancers caused by excessive light exposure (early morning, late evening, and night).
Melatonin restores a disrupted circadian rhythm.
Leilei Wang et al., 2022 Mar – Melatonin Use in Cancer Treatment: Where Are We?
Melatonin inhibits tumor initiation, promotion, and progression by acting on numerous signaling pathways.
Melatonin reduces oncogenic miRNAs while increasing tumor suppressor miRNAs.
Melatonin has the ability to suppress cancer stem cells, which can lead to relapse and metastasis.
Melatonin affects the tumor microenvironment and shifts the immune system response to the cytotoxic side by stimulating the production of cytotoxic T-cells such as CD8 cells or NK cells while inhibiting T regulatory cells (Tregs) and cancer-associated fibroblasts (CAFs), which aid in cancer cell immune escape.
Melatonin improves the effects of chemotherapy while decreasing side effects.
Melatonin makes cancer cells more susceptible to chemotherapy by promoting apoptosis.
Melatonin can also boost the effectiveness of radiation therapy.
Melatonin as an anti-cancer drug has some drawbacks.
With a 45-minute half-life and a 9-33% oral bioavailability,
Melatonin is widely used in clinical practice for the short-term management of sleep disorders such as jet lag or shift work insomnia. It is usually taken orally in doses ranging from 3 mg to 10 mg per day.
Melatonin dosage would be much higher for cancer treatment.
To use melatonin for cancer treatment, the drug form must be stable and have a reasonable shelf life to ensure consistent drug delivery. Melatonin, on the other hand, has a low water solubility and a high permeability.
Melatonin’s low water solubility and poor chemical stability in organic solvents necessitate the development of novel, biocompatible vehicles to improve its bioavailability and solubility.
Nanosized carriers have piqued the interest of researchers due to their unique properties, which include increased therapeutic efficacy, reduced side effects, and improved patient life quality.
Melatonin has been loaded with nanoparticles for cancer treatment.
Melatonin was loaded in 3D-printed magnesium-polycaprolactone to treat osteosarcoma (OS), and it was found to be highly effective in inhibiting OS cell proliferation, invasion, and metastasis both in vitro and in vivo (Zhang et al 2021).
Dosage and safety
Melatonin dosage for adjuvant cancer therapy or cancer prevention has yet to be determined by researchers.
A systematic review compared 50 studies that used melatonin at doses ranging from 0.3 mg to 1600 mg daily for periods ranging from 4 weeks to 3.5 years (15 months on average). There were no adverse events reported in 26 studies (Foley et al 2018).
The most common reported adverse events were psychomotor and neurocognitive dysfunctions, fatigue, and excessive sleepiness, which could be avoided by taking melatonin at night and using the proper dosage with short-term administration. (Foley and colleagues, 2018)
However, when used to treat cancer, relatively high in vitro (0.1-10 mM) and in vivo (5 mg-200 mg/d) doses and long-term duration are required, which may result in severe side effects.
Gonzalez et al., 2021 Jul – Melatonin as an Adjuvant to Antiangiogenic Cancer Treatments
Melatonin has antiangiogenic properties in a variety of tumors.
Melatonin and chemotherapeutic agents work synergistically to inhibit angiogenesis.
Melatonin’s antiangiogenic action in breast cancer may be explained in part by the regulation of pro- and anti-angiogenic miRNAs.
Melatonin has antiangiogenic properties that have been observed in ovarian cancer, prostate cancer, gastric cancer, pancreatic cancer, liver cancer, and colon cancer.
Melatonin inhibits metastatic and angiogenesis in renal carcinoma.
In vitro and in vivo results indicate that combining melatonin with various antitumor drugs and chemotherapeutics improves treatment efficacy.
Melatonin, on its own, has been shown to be an effective inhibitor of proliferation, migration, and invasion.
Many studies have linked melatonin to VEGF inhibition and HIF-1 inactivation, implying that melatonin neutralizes pro-angiogenic and enhances antiangiogenic effects induced by chemotherapeutic agents or radiation.
The conclusion is that the “efficacy of melatonin to sensitize cancer cells to chemotherapeutic agents or ionizing radiation makes it a molecule that can be effective as an adjuvant to these cancer treatments”
Talib et al., 2021 Apr – Melatonin in Cancer Treatment: Current Knowledge and Future Prospects
Melatonin is now recognized as a cell protector as well as a hormone. Melatonin has been shown in studies to be essential in a variety of pathways, including oxidative stress, immune modulation, and hematopoiesis.
The hypothalamic suprachiasmatic nucleus (SCN) is the biological clock that regulates melatonin synthesis and secretion over a 24-hour period.
Melatonin levels rise at night, then fall in the early morning and throughout the day.
Melatonin levels that are elevated at night stimulate target organs to enter into appropriate homeostatic metabolic rhythms, which help to protect the body from the development of various diseases. As a result, exposing the body to light at night may disrupt melatonin production and the circadian rhythm.
Melatonin’s Influence on Cancer Cells
Melatonin helps to maintain genomic stability by scavenging free radicals, inhibiting metal-induced DNA damage, stimulating antioxidant enzymes, improving DNA repair, and inhibiting pro-oxidative enzymes.
Melatonin inhibits cancer cell proliferation (the most important and controlling pathways are hypoxia-inducible factor-1 (HIF-1) signaling, NF-B signaling, PI3K/Akt, insulin-like growth factor receptor (IGF-1R), cyclin-dependent kinases (CDK), and estrogen receptor signaling).
Melatonin induces cancer cell apoptosis (melatonin increases the expression of pro-apoptotic mediators such as BAX/BAK, Apaf-1, caspases, and p53, p38, and p-JNK).
Melatonin prevents angiogenesis.
Melatonin inhibits cancer cell immune evasion – Melatonin can increase T-cell and NK cell production and modulate the immune system.
Melatonin inhibits pro-tumor inflammation.
Melatonin inhibits cancer cell glucose metabolism.
Melatonin inhibits cancer cell metastasis through a variety of mechanisms.
Melatonin can help with the following cancers:
There are numerous pathways for gastric cancer.
Melatonin has been shown to have an anticancer effect in glioblastoma, and it has also been shown to overcome multi-drug resistance in glioblastoma.
Melatonin inhibited the growth and metastasis of prostate cancer cells.
lung cancer (NSCLC) – Melatonin administration significantly increased apoptosis while also inhibiting proliferation, invasion, and metastasis.
Ovarian cancer (involved TLR-4 signaling pathway)
colorectal cancer – reduced proliferation and increased apoptosis
Melatonin promoted autophagy and apoptosis in liver cancer cells.
Melatonin promoted apoptosis and inhibited metastasis in renal cell cancer.
mouth cancer
Melatonin as Adjuvant Treatment to Chemo and Radiation
Melatonin, when used in conjunction with radiotherapy, can enhance the impact of ionizing radiation on tumors while also preventing radiation’s toxic effects on normal cells.
The efficacy of chemotherapy can be improved by taking melatonin at the same time; the latter’s side effects are also reduced.
Melatonin has also been shown to improve survival and quality of life in studies.
Melatonin’s ability to scavenge free radicals, as well as its antioxidant properties, are thought to be responsible for the improved outcomes.
Melatonin Safety Profile and Dosing
Melatonin appears to have a high safety profile based on human trials and reported use, particularly when used in appropriate doses and for a short period of time.
Despite the fact that the doses used in the published studies are 10-50 mg/day higher than those used for other indications (0.5-5.0 mg/d), none of the studies discovered any severe adverse effects associated with melatonin.
Excess sedation and somnolence are the most common side effects.
The half-life of oral melatonin was approximately 45 minutes (28-126 minutes).
Melatonin appears to be safe, as no side effects have been reported, even with doses of up to 100 mg/kg given in 72 hours (Gitto et al) or a dose of 10 mg/kg given once daily for 5 days (Aly et al).
Melatonin, in addition to its poor solubility and stability, has a short plasma half-life, variable oral absorption, and low variable bioavailability.
As a result, traditional oral dosage forms (immediate release) are unsuitable for melatonin delivery.
Many pharmaceutical formulations have been developed using various approaches to overcome these limitations.
My Opinion…
I recently covered high dose Ivermectin and Fenbendazole for the possible treatment of very aggressive COVID-19 mRNA Vaccine Turbo Cancers, as these patients’ Oncologists have given them no options, and Turbo Cancers are resistant to conventional chemo and radiation.
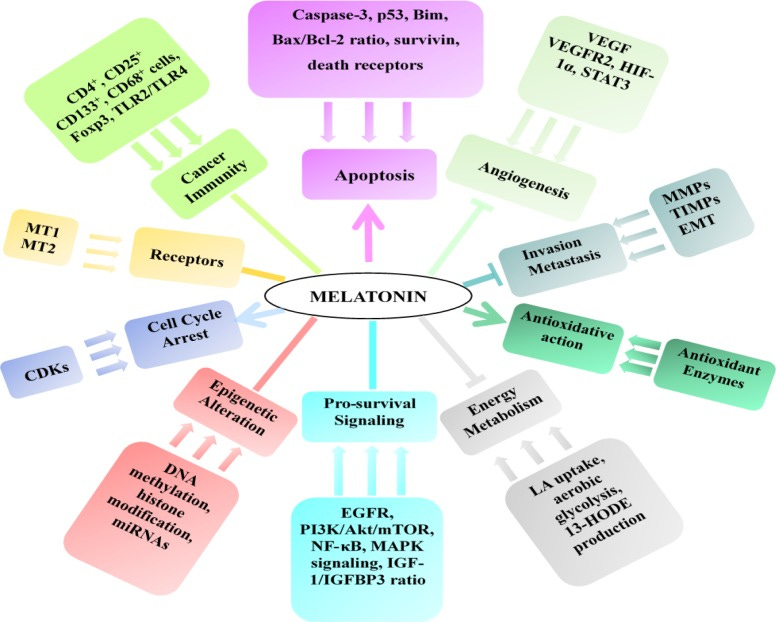
Melatonin has a wide range of anti-cancer properties.
Melatonin’s ability to regulate numerous cancer-related microRNAs, specifically by enhancing tumor suppressor miRNAs and inhibiting oncogenic miRNAs, is particularly impressive in my opinion.
COVID-19 mRNA vaccines may produce oncogenic miRNAs that cause Turbo Cancers (!)
After all, the name of Pfizer’s COVID-19 mRNA Vaccine says it all:
ONCOMIR (Oncogenic miRNAs)
ONCOMIRS, or oncogenic miRNAs, are overexpressed in a variety of cancer types and act via a number of downstream targets. Multiple oncomirs, including the miR-21, miR-31, miR-135, miR-155, and miR-17-92 families, are consistently overexpressed in a variety of cancer types.
These work in a variety of ways to suppress tumor suppressor pathways, activate oncogenic pathways, and even change epigenetic machinery.
In other words, THEY AID CANCER.
What Should You Do With Melatonin?
So, while melatonin has been shown to regulate oncogenic miRNAs in a variety of cancers, can it also regulate Pfizer’s or Co-miRNA-ty’s oncogenic miRNAs?
MAYBE. Nobody has inquired. I’m asking because I don’t know the answer.
Melatonin has been shown to treat breast, colon, lung, hepatobiliary, ovarian, and prostate cancers, which are also the cancers that appear after Pfizer or Moderna COVID-19 mRNA Vaccination, but in Stage 4 Turbo Variety!
Dosing for cancer treatment begins at 20mg per day, but all studies agree that the dose must be much higher. But how high is it?
Some people, presumably safely, take 60mg to 720mg per day:
A Case for Higher Dose Melatonin Integrative Medicine Center of Western Colorado uses 180mg to 720mg per day (see article by William Faloon – A Case for Higher Dose Melatonin).
Melatonin has the ability to boost the immune system, prevent cancer, and treat it. Perhaps it can also be used to treat mRNA Vaccine Turbo Cancer.
However, what are the best doses for each of these indications?
I honestly have no idea.