Pulling Back the Curtain: mRNA Lipid Nanoparticle Design Created Potential for Clotting and Triggering Immune Overdrive
In this series, “Promise or Peril: Alarming COVID-19 mRNA Vaccine Issues,” we look at how the introduction of mRNA technology lacked an adequate regulatory framework, laying the groundwork for serious adverse events and other concerns related to insufficient safety testing of lipid nanoparticles, spike protein, residual DNA and lipid-related impurities, as well as truncated/modified mRNA species.
Previously, we discussed how the FDA relaxed regulations for mRNA vaccines in comparison to mRNA therapies. We also discussed the available data for LNP distribution throughout the body based on animal testing, as well as the absence of human testing. Finally, we discussed the lack of data on the biodistribution of the mRNA and its encoded spike protein in the COVID mRNA vaccine. We will now look at how LNPs are made and how they function in the body. These molecules must be engineered to keep the capsule stable during transit while also allowing it to dissolve quickly once injected.
If the LNPs are overly stable, they may spread throughout the body to distant organs rather than disintegrating locally at the injection site as intended. Other LNP properties, such as electrical charge and clustering tendency, influence the likelihood of adverse events.
Summary of Key Facts:
- The active ingredient in the lipid nanoparticle (LNP) capsule is messenger RNA (mRNA).
- LNP is created by lipids “teaming up” to form a ball.
- LNP molecules have great potential as a delivery vehicle, but their design can be harmful.
- After injection, the LNP capsule may cluster with other LNPs or fall apart, potentially causing clotting.
- Loose strands of mRNA can circulate in the blood if the LNP capsule ruptures.
- Because mRNA is negatively charged, if it clusters with positively charged molecules in the blood, it can cause clotting.
- The lipids in LNP capsules may also cause clotting or cause the immune system to overreact.
- Before the vaccines were approved, researchers were aware of these possibilities.
- Before the drugs were even injected into the body, regulatory agencies were aware of the possibility of adverse effects.
- Before authorization, the possibility of multiple boosters causing harm was also known.
- As time passes, we learn more about the potential mechanisms underlying these adverse events.
Based on laboratory studies and animal models, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approved a novel vaccine product, which was then tested in humans. Furthermore, prior to the pandemic, most mRNA research used intravenous (IV) injection directly into the bloodstream, rather than intramuscular (IM), as vaccines are typically delivered.
Several design challenges had to be overcome in order to create a vaccine based on a repurposed cancer-fighting platform, but some of the LNP’s useful features may be flaws that contribute to adverse events.
LNP Design Features
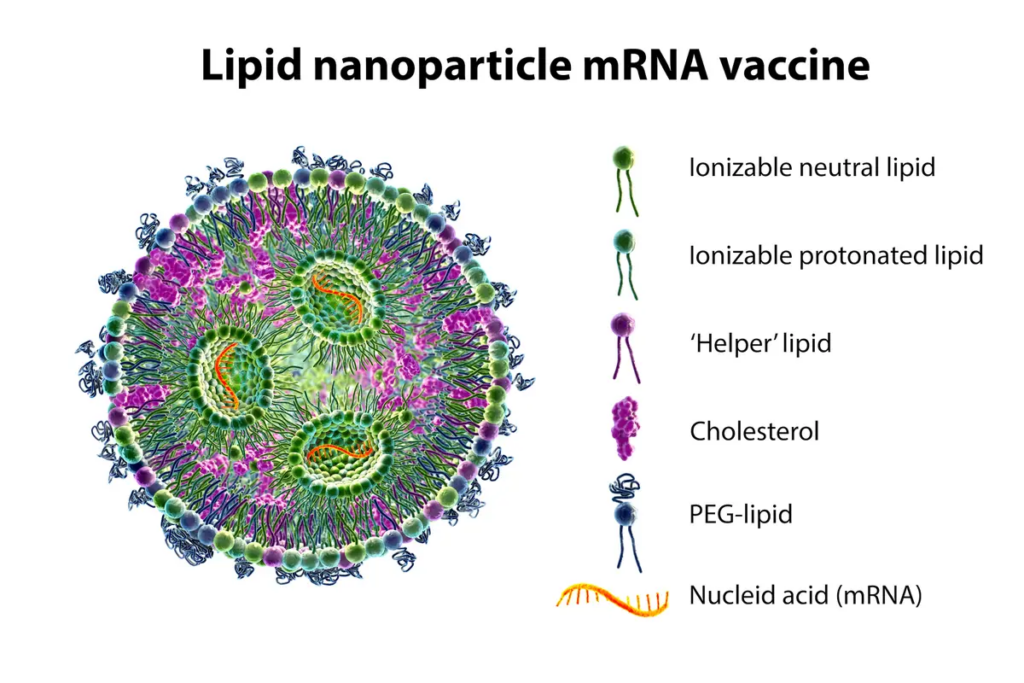
The LNP is a capsule made up of four different lipids that contains the mRNA.
Consider a drop of oil falling into a glass of water. The oil does not disperse in the water; rather, it remains intact. This is how the LNPs stay together while transporting the mRNA to the cell membrane to be absorbed.
Certain lipid properties cause them to organize into the LNP capsule shape. Because of its neutral charge, the lipid tail is hydrophobic, which means it does not mix with water. The lipid’s head is a phosphate with an electrical charge, making it hydrophilic. They organize themselves as a result of these characteristics.
The lipids form a ball with their tails pointing in and their heads pointing out, as shown below. When polyethylene glycol (PEG) binds to a lipid, the PEG-lipid helps to stabilize the molecule, encouraging it to form smaller LNPs and preventing it from adhering to blood proteins.
The RNA, which has a negative charge, is located in the center of the LNP. When the negative charge of the RNA and the positive charge of the phosphate heads on the lipids are added together, the net charge of the LNP is mostly neutral, if not slightly negative.
The PEG-lipids aid in the stability of the LNP. However, once inside the cell, the LNP must split open to release the mRNA cargo. The LNP’s cone-shaped configuration can aid in this process.
Particle size and zeta potential can be affected by the amount of PEG-lipids present. The electrical charge that develops around the surface of a particle is known as zeta potential. The zeta potential is significant because it influences whether LNPs disperse or clump together. A high zeta potential, whether positive or negative, aids in the dispersion and floatability of nanoparticles.
Furthermore, certain other PEG modifications influence how quickly the kidneys and immune system clear the LNPs. If the LNPs take a long time to clear, they can circulate in the blood longer, increasing the risk of adverse events.
LNP Design Dilemmas: Stability Versus Fragility
The LNP design quandary was serious: whether to create a stable LNP capsule that does not easily fall apart or a more fragile capsule that breaks down quickly. The capsule’s behavior in the body is affected by this design challenge.
A highly stable capsule is useful for mRNA gene therapy, which is how this technology was developed originally. In order for gene therapy to work, the mRNA must be stable enough to reach its target and either produce a missing protein or turn off a harmful gene.
However, for vaccination, the opposite effect is desired: the LNP must be less stable so that it dissolves quickly at the injection site and immediately releases the fragile mRNA. Otherwise, the LNP will be able to travel throughout the human body and end up in an unintended organ or tissue.
The biodistribution studies discussed in Parts 1 and 2 show that the LNP mRNA design failed this “dual mission impossible,” with dispersion to distant organs peaking within 48 hours. Because the effect of expressing spike protein on cells in these organs in humans is unknown, simply adopting LNPs designed for gene therapy for direct use in mRNA vaccine delivery is likely to be a significant mistake.
LNP Design Features Affect Clotting
Aside from the challenge of creating a stable LNP that breaks down quickly at the injection site, the LNP design may also cause clustering, which may lead to clotting; if the LNP falls apart, the charges on the lipids and the loose mRNA may promote interactions with other substances in the blood.
These two factors may explain the possibility of “thromboembolic” events. Thrombotic events involve the formation of a clot (thrombosis) in the bloodstream, which may then move to another site (embolism) and block blood flow.
LNPs Can Cluster and Cause Clotting
When LNPs enter the bloodstream, they can grow in size due to the Ostwald ripening phenomenon, which occurs when small crystals dissolve in solution and then redeposit, forming larger clusters.
The diameter of arterioles, which connect arteries and capillaries, ranges from 8000 to 60,000 nanometers (nm). A typical COVID-19 mRNA vaccine LNP is 60 to 200 nm. If the size of the clustered mRNA LNP particles exceeds 5000 nm, LNPs may block blood vessels and cut off blood flow.
When thromboses occur within blood vessels, blood flow to vital organs such as the heart, lungs, kidneys, intestines, and even the brain can be obstructed.
An autopsy review of 25 unexpected deaths within 20 days of COVID-19 vaccination, for example, discovered eight cases of thrombotic events, including five with “myocardial infarction,” two with “pulmonary embolism,” and one with “deep vein thrombosis.”
To our knowledge, no human studies have been conducted to assess the degree to which the LNPs cluster.
The LNP Can Fall Apart
Because of the electrical charge on each component, if the LNP breaks apart, two components, the capsule and the mRNA cargo, may interact to promote clotting.
A positively charged LNP capsule, for example, can target the lung; a negatively charged LNP can target the spleen; and an LNP with an intermediate charge (such as mRNA COVID-19 vaccines) has a greater tendency to travel to the liver, as seen in preclinical biodistribution studies.
The potential for negatively charged free mRNA to cause problems was also seen with AstraZeneca and Johnson & Johnson’s adenovirus vector vaccines, which caused blood clots in some people with a genetic predisposition.
Similarly, if negatively charged mRNA escapes from the LNP carrier, it may cause clotting due to its negative charge.
Could the difficulties in maintaining a strict “cold chain” (freezing temperature required for vaccine stabilization from manufacturing to injection) have introduced the possibility of LNPs falling apart before injection?
“When the LNPs are frozen and thawed,” biotechnology consultant Christie Grace explains, “the [mRNA] can slip out, charges can start interacting with the human body, and [potentially] cause clots.”
Dr. Ko, a South Korean pharmacy professor who has published dozens of articles on LNPs, agrees that if pH and temperature are not carefully controlled, the molecules can break down and separate.
What happens if the LNPs disintegrate in the vial before injection? What testing has been done to evaluate exposed mRNA interactions in the blood (not lipid nanoparticle encapsulated mRNA)?
LNP Engineering Can Alter Clotting
Nanoparticle interactions can be beneficial or detrimental. For example, nanoparticles can be engineered to aid in blood clotting, which is beneficial for those with clotting disorders; however, if LNP interactions with other substances in the blood cause clotting, this is detrimental.
What was known about LNPs’ ability to affect clotting prior to the pandemic?
Faizullin et al. reported in 2020 that “we observed pronounced changes in both clot morphology and kinetics of fibrin clotting in the presence of artificial liposomes.” In other words, previous research on LNPs found that clots looked different and fibrin behaved differently with LNPs.
Fibrin is a component of the human body’s natural clotting cascade, and binding to fibrin speeds up the clotting process. This clotting tendency may be due to the presence of the spike protein’s S1 subunit. Thus, the LNP mRNA vaccine may promote clotting due to the design of the LNP, the presence of the spike protein’s S1 subunit, or both.
‘Immune Overdrive’
Finally, the mRNA was engineered to bypass our natural immune defenses, but this clever design feature may be fatal.
To detect invading microbes, our immune system looks for special patterns, one of which is foreign RNA. To avoid being detected before the vaccine has a chance to work, one part of the COVID-19 vaccine mRNA, uridine, was replaced with N1-methylpseudouridine.
However, if the immune system never notices, we will not receive the intended benefit. Adjuvants, such as aluminum, are added to vaccines for this reason—to stimulate the immune system, which then increases the production of antibodies and memory T cells.
Although the lipids used to make the LNP capsule may stimulate the immune system via the same pattern detectors used to detect harmful invaders, mouse models suggest that LNPs may cause the immune system to go into “overdrive.”
In its report, the EMA noted that the innate immune system ramps up immediately after injection, peaks at six hours, and then returns to baseline nine days later. An article in Cell also discussed the innate immune system in the context of vaccine adverse events (AEs), noting that “frequent booster immunizations may increase the frequency and/or severity of the reported AEs.”
What Was Known Prior to Authorization?
Prior to the approval of the COVID-19 vaccines, early research on LNPs suggests that the following issues were well-documented:
1) The charge of the LNP determines off-target travel throughout the body.
2) LNPs activate the innate immune system, which could result in an overreaction.
3) The immune stimulation is linked to cationic (positively charged) lipid particles.
4) The mode of delivery (muscle or bloodstream) influences where the LNPs travel.
5) As discussed in a previous Epoch Times article, the LNPs were specifically designed for lymphatic uptake.
These side effects were known prior to FDA approval and strongly suggest that more human testing should have been done.
Carrasco et al. appear to share our concerns about the need for a better understanding of human biodistribution, noting that “a specific and important application of these new insights is in the reduction of systemic distribution and off-target expression after IM vaccine delivery.”
Knowledge about charged particle trafficking throughout the body is limited and primarily based on intravenous (IV) injections; prior to the pandemic, only one study published looked at how an intramuscular injection would affect LNP dispersion.
The importance of careful design is summed up in a 2021 Nature article, which notes, as did the EMA, that negatively charged LNPs concentrate in the liver following injection. “This undesirable systemic off-target expression of mRNA-LNP vaccines could be minimized through appropriate design of the ionizable lipid and LNP.”
When we lift the veil on the LNP design, we see that several features intended for stealth delivery of mRNA to the cell have set the stage for a wide range of adverse events that should have been anticipated through testing and avoided through prudent policy.
Part 1: FDA Oversight Required for New Vaccines and mRNA Therapies
Part 2: The Health Consequences of Poor COVID-19 mRNA Testing: Miscarriage, Vision Loss, and Immunotoxicity
Part 4 delves into the cargo contained within the LNP capsule—the mRNA and its encoded spike protein—and how the spike protein and its S1 subunit might impact the cardiovascular system, as well as how recent research suggests that an overactive natural response (cytokines) may cause myocarditis.
◊References
Addgene. Molecular Biology Reference. https://www.addgene.org/mol-bio-reference/#introduction
Alana F Ogata, Chi-An Cheng, Michaël Desjardins, Yasmeen Senussi, Amy C Sherman, Megan Powell, Lewis Novack, Salena Von, Xiaofang Li, Lindsey R Baden, David R Walt, Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Antigen Detected in the Plasma of mRNA-1273 Vaccine Recipients, Clinical Infectious Diseases, Volume 74, Issue 4, 15 February 2022, Pages 715–718, https://doi.org/10.1093/cid/ciab465
Aldén M, Olofsson Falla F, Yang D, Barghouth M, Luan C, Rasmussen M, De Marinis Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr Issues Mol Biol. 2022 Feb 25;44(3):1115-1126. doi: 10.3390/cimb44030073. PMID: 35723296; PMCID: PMC8946961. https://pubmed.ncbi.nlm.nih.gov/35723296/
Anderson EJ, Rouphael NG, Widge AT, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults N Engl J Med 2020; 383:2427-2438 https://www.nejm.org/doi/full/10.1056/NEJMoa2028436
Anderson S. CBER Plans for Monitoring COVID-19 Vaccine Safety and Effectiveness. https://stacks.cdc.gov/view/cdc/97349 October 20, 2020. Accessed 3/20/23.
Angeli F, Spanevello A, Reboldi G, Visca D, Verdecchia P. SARS-CoV-2 vaccines: Lights and shadows. Eur J Intern Med. 2021 Jun;88:1-8. doi: 10.1016/j.ejim.2021.04.019. Epub 2021 Apr 30. PMID: 33966930; PMCID: PMC8084611. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8084611/#bib0043
Baker, A. T., Boyd, R. J., Sarkar, D., Teijeira-Crespo, A., Chan, C. K., Bates, E., Waraich, K., Vant, J., Wilson, E., Truong, C. D., Lipka-Lloyd, M., Fromme, P., Vermaas, J., Williams, D., Machiesky, L., Heurich, M., Nagalo, B. M., Coughlan, L., Umlauf, S., Chiu, P. L., … Borad, M. J. (2021). ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Science Advances. 7(49), eabl8213. https://doi.org/10.1126/sciadv.abl8213
Baumeier C, Aleshcheva G, Harms D, Gross U, Hamm C, Assmus B, Westenfeld R, Kelm M, Rammos S, Wenzel P, Münzel T, Elsässer A, Gailani M, Perings C, Bourakkadi A, Flesch M, Kempf T, Bauersachs J, Escher F, Schultheiss H-P. Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. International Journal of Molecular Sciences. 2022; 23(13):6940. https://doi.org/10.3390/ijms23136940
Bloom, K., van den Berg, F. & Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther 28, 117–129 (2021). https://doi.org/10.1038/s41434-020-00204-y. https://www.nature.com/articles/s41434-020-00204-y (only need one link)
Carrasco, M.J., Alishetty, S., Alameh, MG. et al. Ionization and structural properties of mRNA lipid nanoparticles influence expression in intramuscular and intravascular administration. Commun Biol 4, 956 (2021). https://doi.org/10.1038/s42003-021-02441-2
Chauhan, H., Mohapatra, S., Munt, D.J. et al. Physical-Chemical Characterization and Formulation Considerations for Solid Lipid Nanoparticles. AAPS PharmSciTech 17, 640–651 (2016). https://doi.org/10.1208/s12249-015-0394-x
Chui CSL, Fan M, Wan EYF, et al. Thromboembolic events and hemorrhagic stroke after mRNA (BNT162b2) and inactivated (CoronaVac) covid-19 vaccination: A self-controlled case series study. Lancet. 2022;(50). https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(22)00234-6/fulltext
Dag Berild J, Bergstad Larsen V, Myrup Thiesson E, et al. Analysis of Thromboembolic and Thrombocytopenic Events After the AZD1222, BNT162b2, and MRNA-1273 COVID-19 Vaccines in 3 Nordic Countries. JAMA Netw Open. 2022;5(6):e2217375. doi:10.1001/jamanetworkopen.2022.17375 https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2793348
daSilva RL. Viral-associated thrombotic microangiopathies. Hematology/Oncology and Stem Cell Therapy. 2011:4(2):51-59. https://www.sciencedirect.com/science/article/pii/S165838761150038X
De A, Ko YT. Why mRNA-ionizable LNPs formulations are so short-lived: causes and way-out. Expert Opin Drug Deliv. 2023 Feb;20(2):175-187. doi: 10.1080/17425247.2023.2162876. Epub 2023 Jan 1. PMID: 36588456. https://pubmed.ncbi.nlm.nih.gov/36588456/
Ehaideb, S.N., Abdullah, M.L., Abuyassin, B. et al. Evidence of a wide gap between COVID-19 in humans and animal models: a systematic review. Crit Care 24, 594 (2020). https://doi.org/10.1186/s13054-020-03304-8
European Medicines Agency https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf
Faizullin D, Valiullina Y, Salnikov V, Zuev Y. Direct interaction of fibrinogen with lipid microparticles modulates clotting kinetics and clot structure. Nanomedicine. 2020 Jan;23:102098. doi: 10.1016/j.nano.2019.102098. Epub 2019 Oct 23. PMID: 31655206. https://pubmed.ncbi.nlm.nih.gov/31655206/
FDA. Considerations for Human Radiolabeled Mass Balance Studies – Guidance for Industry. https://www.fda.gov/media/158178/download May, 2022.
FDA. Development and Licensure of Vaccines to Prevent COVID-19. https://www.fda.gov/media/139638/download
FDA-CBER-2021-5683-0013962 approved on: 09-Nov-2020. A Tissue Distribution Study of a [3H]-Labeled Lipid Nanoparticle-mRNA Formulation Containing ALC-0315 and ALC-0159 Following Intramuscular Administration in Wistar Han Rats. FINAL REPORT Test Facility Study No. 185350 Sponsor Reference No. ALC-NC-0552 https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M4_4223_185350.pdf
Fertig TE, Chitoiu L, Marta DS, Ionescu VS, Cismasiu VB, Radu E, Angheluta G, Dobre M, Serbanescu A, Hinescu ME, Gherghiceanu M. Vaccine mRNA Can Be Detected in Blood at 15 Days Post-Vaccination. Biomedicines. 2022 Jun 28;10(7):1538. doi: 10.3390/biomedicines10071538. PMID: 35884842; PMCID: PMC9313234. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9313234/
Grobbelaar LM et al. SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19 Biosci Rep (2021) 41 (8): BSR20210611. https://doi.org/10.1042/BSR20210611
Hassett, KJ, Benenato KE, Jacquinet E, et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Molecular Therapy: Nucleic Acids. 2019;15:P1-11. https://doi.org/10.1016/j.omtn.2019.01.013
Hou, X., Zaks, T., Langer, R. et al. Lipid nanoparticles for mRNA delivery. Nat Rev Mater 6, 1078–1094 (2021). https://doi.org/10.1038/s41578-021-00358-0
Let’s talk about lipid nanoparticles. Nat Rev Mater 6, 99 (2021). https://www.nature.com/articles/s41578-021-00281-4
Michieletto, D., Lusic, M., Marenduzzo, D. et al. Physical principles of retroviral integration in the human genome. Nat Commun 10, 575 (2019). https://doi.org/10.1038/s41467-019-08333-8
Moghimi, S. M., & Simberg, D. (2022). Pro-inflammatory concerns with lipid nanoparticles. Molecular therapy : The Journal of the American Society of Gene Therapy, 30(6), 2109–2110. https://doi.org/10.1016/j.ymthe.2022.04.011
Naturally Inspired Podcast. Jessica Rose PhD – VAERS, Data And Truth https://www.audible.com/pd/Jessica-Rose-PhD-VAERS-Data-And-Truth-Podcast/B09YMLJGBN?clientContext=132-5166709-6339436&loginAttempt=true&noChallengeShown=true
Ohlson J. Plasmid manufacture is the bottleneck of the genetic medicine revolution. Drug Discov Today. 2020 Oct 16;25(11):1891–3. doi: 10.1016/j.drudis.2020.09.040. Epub ahead of print. PMID: 33075470; PMCID: PMC7564888. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7564888/
Perico L, Marina Morigi M, Galbusera M, et al. SARS-CoV-2 Spike Protein 1 Activates Microvascular Endothelial Cells and Complement System Leading to Platelet Aggregation. Front. Immunol. 2022 https://www.frontiersin.org/articles/10.3389/fimmu.2022.827146/full
Qin, S., Tang, X., Chen, Y. et al. mRNA-based therapeutics: powerful and versatile tools to combat diseases. Sig Transduct Target Ther 7, 166 (2022). https://doi.org/10.1038/s41392-022-01007-w
Röltgen K, Nielsen SCA, Silva O. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022;185(6):1025-1040. https://www.cell.com/cell/fulltext/S0092-8674(22)00076-9
Schmeling, M, Manniche, V, Hansen, PR. Batch-dependent safety of the BNT162b2 mRNA COVID-19 vaccine. Eur J Clin Invest. 2023; 00:e13998. doi:10.1111/eci.13998 https://pubmed.ncbi.nlm.nih.gov/36997290/
Srinivasan M, Thangaraj SR, Arzoun H. Gene Therapy – Can it Cure Type 1 Diabetes? Cureus. 2021 Dec 19;13(12):e20516. doi: 10.7759/cureus.20516. PMID: 35004071; PMCID: PMC8723777. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8723777/
Trevaskis, N., Kaminskas, L. & Porter, C. From sewer to saviour — targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov 14, 781–803 (2015). https://doi.org/10.1038/nrd4608
Trougakos IP, Terpos E, Alexopoulos H, et al. Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Cell 2022;28(7): P542-554. https://www.cell.com/trends/molecular-medicine/fulltext/S1471-4914(22)00103-4
Vervaeke P, Borgos SE, Sanders NN, Combes F. Regulatory guidelines and preclinical tools to study the biodistribution of RNA therapeutics. Adv Drug Deliv Rev. 2022 May;184:114236. doi: 10.1016/j.addr.2022.114236. Epub 2022 Mar 26. PMID: 35351470; PMCID: PMC8957368. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8957368/
Wong DWL, Klinkhammer BM, Djudjaj S, Villwock S, Timm MC, Buhl EM, Wucherpfennig S, Cacchi C, Braunschweig T, Knüchel-Clarke R, Jonigk D, Werlein C, Bülow RD, Dahl E, von Stillfried S, Boor P. Multisystemic Cellular Tropism of SARS-CoV-2 in Autopsies of COVID-19 Patients. Cells. 2021 Jul 27;10(8):1900. doi: 10.3390/cells10081900. PMID: 34440669; PMCID: PMC8394956. https://pubmed.ncbi.nlm.nih.gov/34440669/
Yonker LM, Swank Z, Bartsch YC, et al. Circulating Spike Protein Detected in Post–COVID-19 mRNA Vaccine Myocarditis. Circulation. 2023:147(11). https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.122.061025